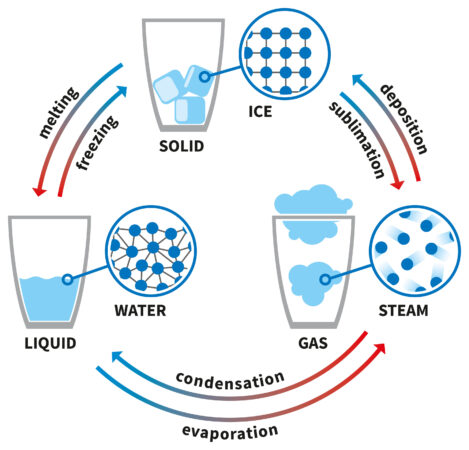
Ice, water and vapor are three clearly different kinds– or states– of water. Like various other materials, water can take different types as its surrounding environment changes. Take, for instance, an ice-cube tray. Put water into the tray, stick it in the fridge freezer and also a few hours later that fluid water will have transformed right into solid ice. The compound in the tray is still the exact same chemical– H2O; only its state has transformed.
Put the ice into a pot over a flame on the stove as well as it will melt pull back to fluid. If it gets hot enough, you will certainly see vapor increasing off of the liquid. This vapor is still H2O, just in gas form. Solid (the ice), fluid (the water) and gas (the vapor) are the 3 most typical states of matter— a minimum of on Earth.
In old Greece, one philosopher identified just how water could alter kind and also reasoned that every little thing needs to be constructed from water. Nonetheless, water isn’t the only kind of issue that transforms states as it’s warmed, cooled down or pressed. All matter is made of atoms and/or particles. When these tiny foundation of issue transform their framework, their state or phase does as well.
)Also today, researchers are still finding brand-new states of issue. While there’s likely much more waiting for discovery, below are seven of the currently agreed-upon states that matter can take.
Strong: Materials in this state have a precise volume and form. That is, they use up a collection amount of area. And also they’ll preserve their shape without the help of a container. A desk, phone as well as tree are all instances of matter in its strong kind.
The atoms and molecules that make up a strong are tightly compacted. They’re so tightly bound that they do not move freely. A strong might melt into a liquid. Or it could sublimate– transform directly from solid to gas when brought to certain temperatures or pressures.
Liquid: Materials in this state have a precise quantity but no defined form. Squeezing a liquid will not compress it right into a smaller sized volume. A fluid will take the shape of any kind of container into which it’s put. Yet it will certainly not broaden to fill up the whole container holding it. Water, shampoo and milk are all instances of liquids.
A fluid’s atoms and particles are less snugly loaded than those in a strong. A liquid could be cooled right into a strong. When heated sufficient, it will usually become a gas.
Within one of the most typical stages of issue, various other states might show up. For example, there are liquid crystals. They appear to be a fluid as well as flow like a fluid. Their molecular structure, however, better resembles strong crystals. Soapy water is an instance of an usual liquid crystal. Numerous tools take advantage of liquid crystals, consisting of cellular phone, TVs and also digital clocks.
Gas: Materials in this stage have no guaranteed quantity nor shape. A gas will both take the form of its container and increase to fill that container. Examples of common gases consist of helium (made use of to make balloons float), the air we breathe as well as the natural gas made use of to power many kitchen ranges.
The atoms and particles of a gas likewise move much more quickly as well as freely than those in a solid or fluid. The chemical bonds between the molecules in a gas are very weak. Those atoms and molecules are also farther apart than those of the exact same material in its fluid or strong types. When cooled down, a gas may condense into a liquid. For example, water vapor in air can condense outside a glass holding cold water. This can develop little water beads. They can run down the side of the glass, developing small pools of condensation on a table top. (That’s one factor individuals utilize coasters for their beverages.)
The word “liquid” can refer to a liquid or a gas. Some fluids are supercritical. This is a state of issue that happens at a crucial point of temperature level as well as stress. At this point, fluids and also gases can not be told apart. Such supercritical liquids take place naturally in the ambiences of Jupiter and Saturn.
Plasmas are frequently discovered in celebrities, including our sun. A plasma can be created by heating a gas to extremely high temperatures. A plasma might additionally create when a shock of high voltage crosses an area of air in between two factors. Though they are uncommon on Earth, plasmas are one of the most typical type of matter in deep space.
it(tip: virtually everywhere)and also what makes it so special. Bose-Einstein condensate: A very-low-density gas that has been cooled to near absolute no changes right into a new state of matter: a Bose-Einstein condensate. Absolute zero is thought to be the most affordable temperature possible: 0 kelvin,– 273 levels Celsius or about– 459.67 levels Fahrenheit. As this low-density gas gets into such a super-cold program, all of its atoms will ultimately begin to “condense” right into the exact same power state. Once they reach it, they will currently serve as a “superatom.” A superatom is a cluster of atoms that act as if they were a single bit.
Bose-Einstein condensates do not create normally. They develop just under meticulously managed, extreme laboratory conditions.
Degenerate issue: This state of issue creates when a gas is super-compressed. It currently starts to act even more like a solid, even though it continues to be a gas.
Normally, atoms in a gas will relocate swiftly and openly. Not so in degenerate (Deh-JEN-er-ut) issue. Below, they are under such high stress that the atoms smoosh carefully with each other right into a little room. As in a strong, they can no longer move freely.
Stars at the end of their lives, such as white towers over and neutron celebrities, consist of degenerate matter. It’s what enables such stars to be so little as well as dense.
There are several different sorts of degenerate issue, including electron-degenerate matter. This type of matter contains primarily electrons. An additional instance is neutron-degenerate matter. That form of issue consists of mainly neutrons.
Quark-gluon plasma: As its name suggests, a quark-gluon plasma is made up of the fundamental particles referred to as quarks and also gluons. Quarks come together to form fragments like protons and neutrons. Gluons act as the “adhesive” that holds those quarks together. A quark-gluon plasma was the first kind of issue to fill the universe complying with the Big Bang.
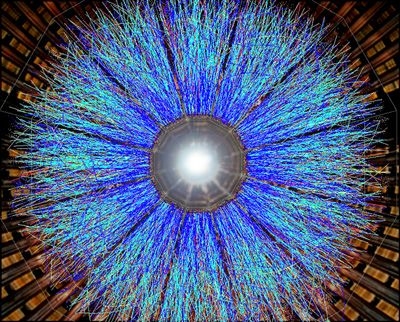
European Organization for Nuclear Study, or CERN, very first detected a quark-gluon plasma in 2000. After that, in 2005, scientists at Brookhaven National Research Laboratory in Upton, N.Y., developed a quark-gluon plasma by smashing gold atoms together at close to the rate of light. Such energetic crashes can produce intense temperatures– approximately 250,000 times hotter than the sun’s inside. The atom smashups were hot enough to break down the protons and neutrons in the atomic centers into quarks and also gluons.
It had actually been expected that this quark-gluon plasma would certainly be a gas. But the Brookhaven experiment revealed it was actually a sort of fluid. Ever since, a series of experiments have actually revealed that the plasma works as a super-liquid, exhibiting less resistance to circulation than any other compound.
A quark-gluon plasma when filled up the entire cosmos– like a sort of soup– where matter as we know it arised.
As well as a lot more? As with liquid crystals as well as supercritical fluids, there are much more states of matter than those described over. As scientists proceed working to comprehend the world around us, they will likely keep finding newer as well as stranger manner ins which atoms, that make up whatever worldwide around us, act under extreme problems.

