Imagine a device package for snapping together molecules like Lego foundation. That’s basically the advancement that brought three drug stores this year’s Nobel Reward in chemistry.
The Royal Swedish Academy of Sciences in Stockholm introduced the award at a news conference on October 5.
With her win, Carolyn Bertozzi of Stanford College in The golden state comes to be only the 8th female to take home a Nobel in this field. She shares it with Morten Meldal at the College of Copenhagen in Denmark and Barry Sharpless of the Scripps Study Institute in La Jolla, Calif. The triad won for introducing what’s being called click chemistry. Meldal’s as well as Sharpless’ “click tool” permits scientists to easily develop intricate particles in the laboratory. Bertozzi’s job enabled it to also be made use of inside living microorganisms.
Explainer: The Nobel Prize
“The advantage with this exploration is that it can be utilized for nearly every little thing,” says Olof Ramström. He’s a chemist at the College of Massachusetts Lowell as well as a member of the Nobel board for chemistry. The new techniques can be utilized to build drug molecules, polymers as well as other brand-new materials. They likewise can be made use of to track chosen molecules in the body.
“I assume this chemistry is going to transform medicine in so many locations,” states Angela Wilson of Michigan State College in East Lansing. (She’s also head of state of the American Chemical Culture, in Washington D.C.) In regards to using these methods, she notes, “We’re kind of at the idea of the iceberg.”
Around twenty years ago, Sharpless introduced the concept of click chemistry. It’s a method to simply and also quickly affix two substances using specific connector particles. But finding Lego-like ports to affix those building blocks wasn’t simple. On their own, Sharpless and Meldal each uncovered the remedy. They included a smidge of copper to a mixture consisting of two various other tiny particles. This allowed the scientists swiftly snap the two small molecules with each other into a ring-shaped chemical.
This response promptly “acquired substantial interest,” Ramström notes. Even though scientists would certainly later find a handful of various other particles that might snap together in the same fashion, that first response is considered the “crown gem of click reactions.”
While triggering those reactions with copper might function great in a glass beaker, that metal can harm living cells. Bertozzi uncovered a means to do copper-free click chemistry. Now scientists can utilize it to make certain chain reactions happen in living things– without mucking up their cells’ normal activities.
Making click chemistry risk-free for cells
Bertozzi’s specialty has been examining sugar molecules. These “are extremely difficult to work with,” explains Leslie Vosshall. She’s a neuroscientist at Rockefeller College in New York City. She additionally is the vice head of state as well as principal clinical policeman at the Howard Hughes Medical Institute. Straight approaches exist for taking a look at DNA, RNA as well as healthy proteins. The very same has not been true for sugars, she keeps in mind. “Sugars are the dark issue of the cell.”
Bertozzi’s Nobel-winning technology was to method cells right into including a click chemical right into the sugars that adorn their external surface. Now, when researchers reveal these cells to a different click chemical, both will break with each other. It’s similar to the particles carry out in Sharpless’ and also Meldal’s reactions. By linking cells to green-glowing molecules, as an example, scientists can now make the cells’ surface areas radiance.
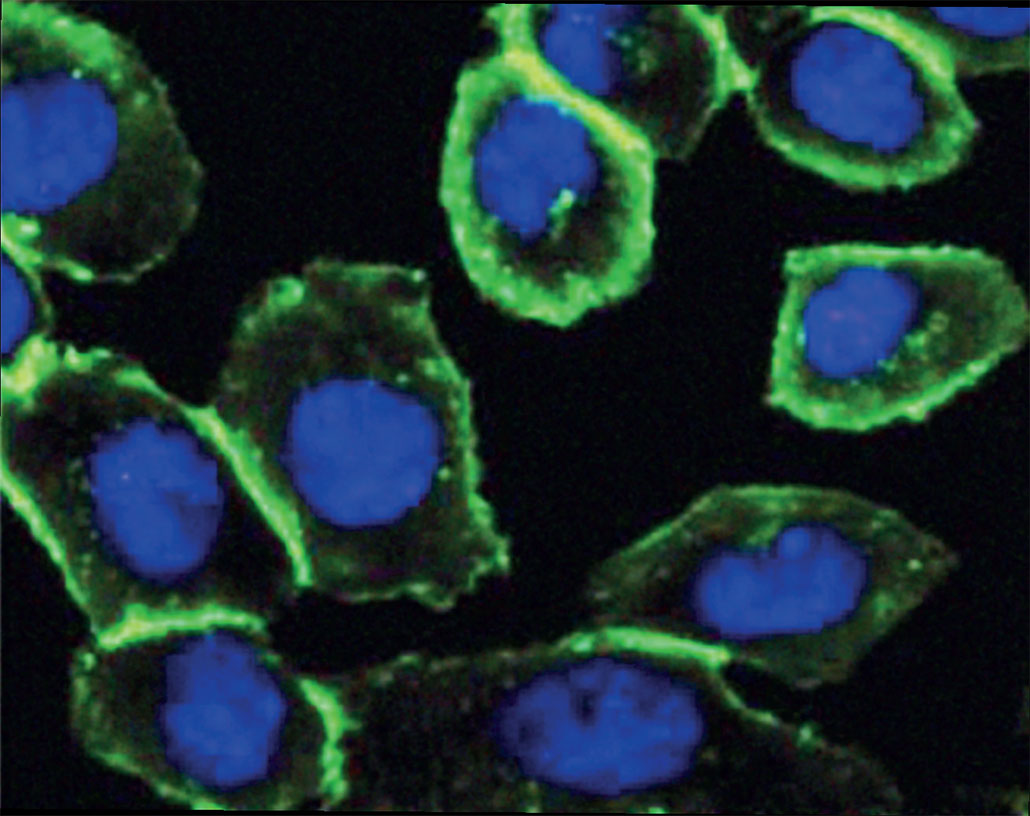
they are and how they move,”notes Johan Åqvist.”This is what Carolyn Bertozzi did,”the chemist explains. Åqvist works at Uppsala University in Sweden. He likewise chairs the Nobel committee for chemistry. By targeting specific sugars on cell surfaces, researchers can create brand-new treatments. As an example, Bertozzi and her associates were able to target– and also shut off– sugars that can aid growth cells
conceal from the immune system’s T cells.”Carolyn is … one of the amazingly few women in chemical biology, “states Vosshall. Her lab”has influenced ladies drug stores as well as put them out right into the globe.”Bertozzi got up to a 3 a.m. phone call signaling her she was a Nobel victor.
On listening to the information, she remembers being”absolutely stunned. I’m resting right here as well as can rarely take a breath. “Stating this middle-of-the-night phone call was a shock is an understatement, she added.”I’m still not totally positive that it’s genuine. Yet it’s getting realer by the min. “Bertozzi, Meldal and Sharpless will share 10 million Swedish kronor.( That deserves about$900,000.)The win is the 2nd for Sharpless. He additionally shared the 2001 Nobel prize in chemistry. That win was for his service a new type of stimulant.

