The Neuse River waterdog may live more than 50 years. But it never grows up.
This brown-and-black salamander lurks in rivers and creeks of North Carolina. Like its close cousins, frogs and toads, it is an amphibian. It begins life as a tadpole, before growing legs. But unlike its hopping relatives, the waterdog never outgrows its larval body.
Its tail always remains finned, like a tadpole’s. Its clumsy little legs never grow large enough for its body. Its rear feet never finish sprouting their toes. And it spends its whole life breathing through larval gills. They jut out from its neck like puffy peacock feathers. Because the waterdog never grows up, it must spend its entire life in water.
It also moves slowly — as if its fat little body was stuffed full of lead. In fact, it really is stuffed. Each cell in its body is crammed full of DNA — 38 times as much DNA as a human cell contains.

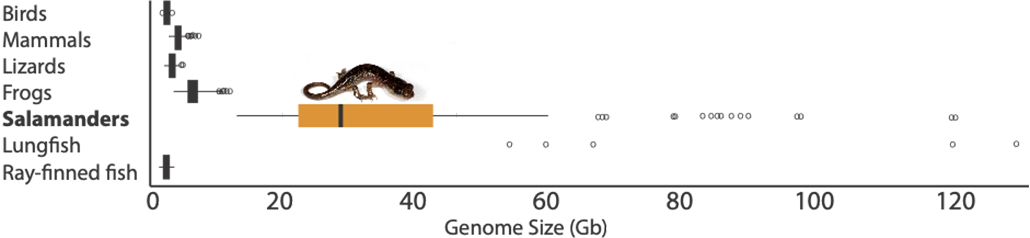
An animal’s genome is the complete set of DNA in each of its cells. Each molecule of DNA is shaped like a very long ladder. And each rung on the ladder is known as a “base pair.” The Neuse (Noose) River waterdog has the largest genome of any four-legged beast on Earth. The genomes of most mammals, birds, reptiles and fish fall within a narrow size range — roughly one billion to four billion base pairs. But salamander genomes range more wildly — from 10 billion up to 120 billion base pairs.
This huge load of DNA has utterly warped salamanders’ bodies. It keeps many species from ever reaching adulthood. It leaves others with simplified brains, poor eyesight and bendy bones that never harden.
“Big genomes are just part and parcel of what salamanders are,” said biologist David Wake. “They are living, walking embryos.” Prior to his death last year, he spent decades at the University of California, Berkeley, studying salamanders.
DNA is often called the blueprint of life. Genes in our DNA determine whether we have feathers, scales or hair. They guide how our blood vessels branch and our nerve cells connect. But salamanders are revealing that DNA also shapes us in ways that have nothing to do with genes or the data they hold. Like the creamy filling in a Twinkie, DNA expands to fill as much space as it can. Every species struggles to keep its DNA from growing too much. This struggle has shaped every animal — from aardvarks to zebrafish — and likely all other life as well.

Parasites run amok
The mystery of salamanders began in the mid-20th century. Scientists had only just shown that DNA was the hereditary molecule of life. They had shown it contains the genes that get passed down from one generation to the next.
In 1950, people had some strong beliefs about how the human genome would stack up against others.
“We think we’re the most complex animals on the planet,” says T. Ryan Gregory. That suggested, he says, “We should have the most genes. We should have the most DNA.” That assumption turned out to be very wrong. Gregory is a biologist who studies genome size at the University of Guelph in Ontario, Canada.
In 1951, two scientists measured the DNA in cells from 54 species of fish, amphibians, reptiles, mammals and birds. Salamanders had dozens of times more DNA than humans and other animals. Lungfish — strange fish that can walk on their fins and breathe air — also had huge amounts of DNA. The pair published its discovery in the Journal of General Physiology. Such giant genomes have intrigued scientists ever since.
DNA ‘parasites’
Over the next half century, people gradually realized that salamanders and lungfish do not actually have more genes than other animals.
DNA contains more than genes. And some of its other stuff has no clear function. Scientists sometimes call this extra stuff junk DNA. Among this non-gene DNA are things known as transposons (Trans-POH-zahns). Salamanders and lungfish have way more junk DNA than other animals — and especially more of those transposons.
Think of transposons as DNA parasites.
Parasites are typically small critters that live in or on the bodies of larger beasts. Among better known ones are tapeworms, hookworms, lice and ticks. They often harm their hosts, making them less healthy. Transposons operate like parasites of the genome. These snippets of DNA constantly make new copies of themselves, which they then insert into a host’s genome.
Some ecologists value parasites — and now want a plan to save them
And that can sometimes harm the host. If these parasites end up in the middle of some gene, they might stop it from working properly. And this might cause cancer — or birth defects, where animals are born with missing limbs, abnormal hearts or other problems.
To try and avoid this problem, all animals have special proteins that work like an immune system for the genome. These proteins make it harder for transposons to copy themselves. But in 2011, Rachel Mueller discovered that something had gone horribly awry in salamanders. Their transposons had multiplied out of control.
Mueller is an evolutionary biologist at Colorado State University in Fort Collins. She sequenced DNA from six species of salamanders. And dozens of different transposons in salamanders showed up at high levels compared to those in frogs, zebrafish, people and other animals. One of these invaders, called Ty3/gypsy, may have had more than a million copies inserted into the salamander genomes. Mueller and her team shared their findings in Genome Biology and Evolution.
No one knows why transposons multiplied out of control in salamanders. But that extra DNA has slowed their lives and warped their bodies.

Perpetual babies
Salamanders develop slowly because the genes that guide their growth take a long time to turn on and become active. For a gene to turn on, its entire length must be copied into a messenger molecule, called RNA. Longer genes take more time to copy. Because salamander genes are stuffed full of transposons, they are often five times as long as human genes. As a result, important signals that guide the growth of body parts can be delayed in salamanders.
“Salamanders are basically babies walking around,” concludes Stanley Sessions. “They never quite grow up.” He is a retired salamander biologist at Hartwick College in Oneonta, N.Y.
Salamanders with the largest genomes — such as the waterdog — never finish metamorphosis. That’s the process of changing from tadpoles into land-dwelling adults.
Some of these species don’t even finish sprouting their limbs and toes. Consider the amphiuma (Am-fee-OOM-ah) family. Most salamanders have four toes on their front feet and five on their rear. But amphiumas have fewer — sometimes just one toe on each foot. Their legs are so tiny that these animals look like eels. They are often called “conger eels.” And salamanders in another family — sirens — don’t sprout any rear legs.
Many salamanders with smaller genomes do grow into land-dwelling adults with all their legs and toes. But even these don’t reach full maturity, scientists have found.

In some species, the skull bones never finish growing together. This leaves a gap on top of the head. The gap leaves part of the brain unprotected. (Our skulls had a similar gap at birth, but this “soft spot” closes by age three.)
In other salamander species, wrist and foot parts of their skeletons never fully harden. The animal is left with bendy “bones” made of cartilage.
Big cells, blocky bodies
Salamanders are also weird in another important way. Their cells are huge. This was discovered long before anyone knew about their big genomes. A British army surgeon named George Gulliver noticed those big cells in 1875.
He traveled the world with the British army. Gulliver collected blood from hundreds of species and measured the size of their red blood cells. Amphiuma salamanders had cells almost 300 times larger than those of humans, he found.
People now understand that salamanders have big cells because their DNA takes up a lot of space. The bigger the genome, the larger the cell. In some species, you can even see red cells zipping through blood vessels in the gills using just a magnifying glass. (In other animals, you need a powerful microscope to see red blood cells.)
Having large cells changes the way that salamanders are put together.
The animals become “pixelated,” says Sessions. In digital photos, the clarity of images changes when you replace their tiny dots of color — or pixels — with bigger blocks of color. It’s like the face of a person in the computer game Minecraft. The head is blocky, with simple features. The eyes and mouth are just a few squares. And details like lips and eyelashes are left out. Sessions says it’s kind of like that with salamanders.
In some species, the brain is separated from the outside world by only a single layer of skin cells. The femur bones of the leg are sometimes only two or three cells thick.
Explainer: Cells and their parts
In 1994, Wake at UC Berkeley worked with Gerhard Roth, a brain scientist at the University of Bremen in Germany. The two showed that salamanders with bigger cells also have simpler brains. They have fewer cells in the nerve that relays signals from the eyes to the brain. And they have fewer cells in the brain area that receives signals from the eyes.
No one knows for sure what this means for the animals. But Roth does suspect that affected species may have limited vision compared to those with smaller, more numerous brain cells.
Salamanders with bigger genomes and cells also seem to have weaker hearts. Michael Itgen discovered this when he compared the heart structures of nine species. Itgen is a PhD student working with Mueller in Colorado. He has been studying salamanders with genomes from nine to 22 times the size of the human genome.
Those with smaller genomes have hearts with thick, muscular walls, Itgen finds. They may be able to pump blood forcefully. But as the genomes get larger, the hearts become hollower. And their walls thin. Animals with the biggest genomes have flimsy heart walls. The heart muscle is sometimes only one cell thick!
“I was shocked,” says Itgen. Those thin hearts probably can’t pump blood very strongly. He and Mueller described their findings in June in the journal Evolution.
Itgen believes that genome and cell size likely affect the structure of every organ in a salamander’s body. He studied the liver, which removes toxins and damaged red cells from the blood. He found differences in that organ, too.

Tolerating DNA
These effects reveal some of the challenges that come with having so much DNA. And yet salamanders are clearly doing fine. After all, they have survived for 200 million years. Because of this, some people now wonder whether that extra DNA somehow helps the animals.
Sessions thinks it does. Salamanders have an amazing ability to regenerate, he notes. If their legs or tails get torn off, they can regrow new ones. He believes the animals’ huge genomes help here.
Because salamanders develop slowly, Sessions believes that adults still have plenty of embryonic cells. These “stem cells” may possess the ability to mature into any of many specialized tissues, such as muscle, skin or bone. If an animal loses a leg later in life, these cells may help to regrow it.
Lungfish can also regenerate. And like salamanders, lungfish have huge genomes, live slowly and never grow up. “Lungfish are the exception that proves the rule,” says Sessions. He and Wake published this theory last year in Developmental Dynamics.

But there may be yet a deeper reason why salamanders can tolerate huge genomes. They have evolved a way of living that does not require them to move or grow as quickly as other animals. So it makes no difference if their big genomes impose some speed limit on them.
Salamanders have a much slower metabolism than mammals, birds or even cold-blooded reptiles. They do not need nearly as much oxygen. In fact, the salamanders that Itgen and Mueller have studied do not need lungs or gills to get oxygen. That gas seeps straight into their skin from the air.
This probably helps them survive with weak hearts, says Mueller. People depend on strong hearts to pump oxygen from our lungs to the rest of our body. But salamander hearts don’t have to work nearly as hard.
In fact, some salamanders can even survive having a quarter of their heart cut out, Sessions has found. This would quickly kill most animals. But the salamanders lived long enough to grow it back.
Salamanders’ slow movements also help them conserve energy. Many have poisonous skin — so most predators leave them alone. And few salamanders need to chase prey. They just sit still until an insect happens by. Then — zap — they strike with a lightning-quick tongue or a ferocious gulp. “That’s about the only thing they do fast,” said Wake. “But it’s extremely fast.”
The development of ‘salamonsters’
The salamander genome seems odd — but it also reveals an important lesson about all life. Transposons tend to multiply, just as do viruses, bacteria and bunny rabbits. Because transposons multiply, genomes tend to expand over millions of years. The critical question is how much this parasite DNA can expand without hurting its host.
The ancestors of salamanders probably lived and grew slowly, says Mueller. So they weren’t harmed as their transposons multiplied and their genomes expanded. These animals gradually evolved more effective strategies for surviving in the slow lane. This, in turn, might have allowed their genomes to further expand.
Today, salamanders with the largest genomes — such as amphiumas, sirens and waterdogs — live in places where water is always present. This has let their genomes expand until these species lost the ability to morph into land-dwelling adults. These species are “salamonsters,” says Sessions. They “have simply given up” trying to control their transposons.
But for animals that must live a faster life to survive, their bodies have kept their genomes from growing too large.
Birds have genomes smaller than do humans. They have to grow quickly to be able to migrate with the seasons or to follow their food species. Hummingbirds have some of the smallest genomes of all. Some are less than one-third the size of the human genome. That super-fast metabolism powers wings that beat dozens of times per second and hearts that beat up to 1,200 times per second. “There’s just no way you could have a bird with a salamander-sized genome,” concludes Gregory at Guelph.
Among mammals, bats have the smallest genomes, similar in size to those in birds. And because bats fly, they face similar physical challenges.

Most frogs and toads have largish genomes. Although not as big as a salamander’s, some of them are still up to four times the size of the human genome. But in 2021, scientists reported one striking exception. The ornate burrowing frog has a genome about as small as a hummingbird’s.
This frog probably evolved its tiny genome because it had to survive in the desert. It lays eggs in puddles that form, briefly, after rains. Emerging tadpoles have only a few days to grow legs and lungs before these puddles evaporate.
The need for fast development forced this species to get its transposons under control. To do this, it evolved a stronger genomic “immune system.” And over millions of years, it cut the number of transposons in its DNA. The results were published last year in the Proceedings of the National Academy of Sciences.
Even plants follow this grand pattern. Fast-growing weeds like dandelions and thistles tend to have smaller genomes than slower-growing plants. Genome size “is not random,” concludes Gregory.
This tug-of-war between organism and genome has shaped every species. But we might never have known about it, if not for the really weird critters that gave us the first clues. For this, we can thank the sluggish waterdog.